Manufacturing Methode
Manufacturing Methode
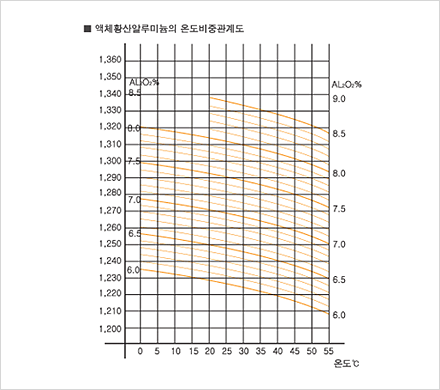
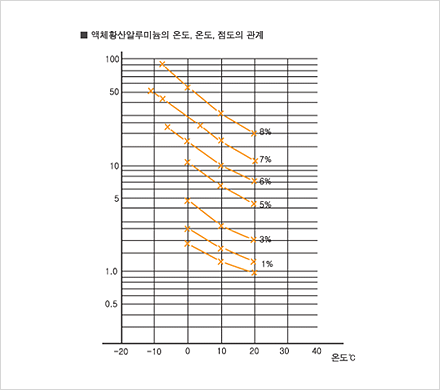
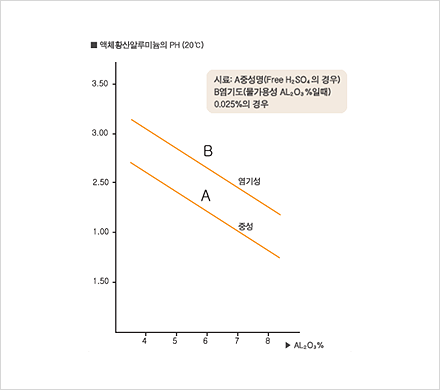
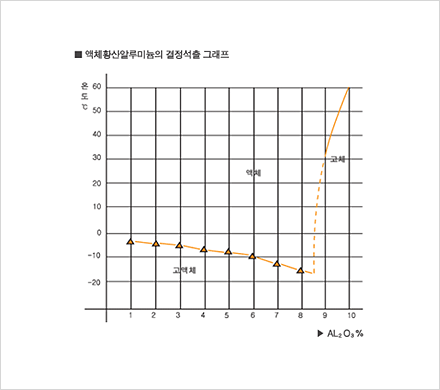
Since 1985, our company has been producing aluminum sulfate and selling it to meet a wide range of demands on different levels. We have adapted well to changes in demand in recent years and to the elevation of quality standards through technological innovation. Due to our consistent quality management effort, we have acquired a KS M1411 certification and are now producing a most reliable product. By market demand, our company is now shifting from producing solid aluminum, sulfate to liquid aluminum sulfate. We are thus extending our manufacturing equipment for liquid aluminium sulfate, even as we are manufacturing products with different particle size.
- Water purifier : national water supply, industrial water supply, industrial waste water, metropolitan sewage
- Die production
- Figment production
- Precipitant for ceramic materials
- Pharmaceutical production
- As a raw material for various aluminum compounds